- Thyroid
- Clinicopathological Features and Molecular Signatures of Lateral Neck Lymph Node Metastasis in Papillary Thyroid Microcarcinoma
-
Jinsun Lim, Han Sai Lee, Jin-Hyung Heo, Young Shin Song
-
Endocrinol Metab. 2024;39(2):324-333. Published online April 4, 2024
-
DOI: https://doi.org/10.3803/EnM.2023.1885
-
-
 Abstract Abstract
 PDF PDF Supplementary Material Supplementary Material PubReader PubReader  ePub ePub
- Background
The predictive factors for lateral neck lymph node metastasis (LLNM) in papillary thyroid microcarcinoma (PTMC) remain undetermined. This study investigated the clinicopathological characteristics, transcriptomes, and tumor microenvironment in PTMC according to the LLNM status. We aimed to identify the biomarkers associated with LLNM development.
Methods
We retrospectively reviewed the medical records of patients with PTMC from two independent institutions between 2018 and 2022 (n=597 and n=467). We compared clinicopathological features between patients without lymph node metastasis (N0) and those with LLNM (N1b). Additionally, laser capture microdissection and RNA sequencing were performed on primary tumors from both groups, including metastatic lymph nodes from the N1b group (n=30; 20 primary tumors and 10 paired LLNMs). We corroborated the findings using RNA sequencing data from 16 BRAF-like PTMCs from The Cancer Genome Atlas. Transcriptomic analyses were validated by immunohistochemical staining.
Results
Clinicopathological characteristics, such as male sex, multifocality, extrathyroidal extension, lymphatic invasion, and central node metastasis showed associations with LLNM in PTMCs. Transcriptomic profiles between the N0 and N1b PTMC groups were similar. However, tumor microenvironment deconvolution from RNA sequencing and immunohistochemistry revealed an increased abundance of tumor-associated macrophages, particularly M2 macrophages, in the N1b group.
Conclusion
Patients with PTMC who have a male sex, multifocality, extrathyroidal extension, lymphatic invasion, and central node metastasis exhibited an elevated risk for LLNM. Furthermore, infiltration of M2 macrophages in the tumor microenvironment potentially supports tumor progression and LLNM in PTMCs.
- Thyroid
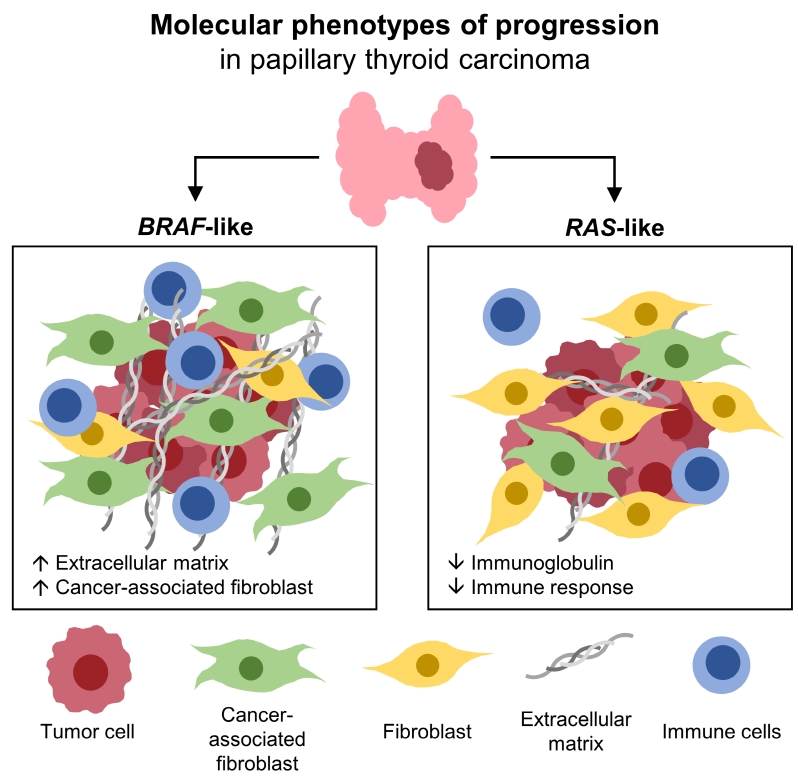
- Different Molecular Phenotypes of Progression in BRAF- and RAS-Like Papillary Thyroid Carcinoma
-
Jinsun Lim, Han Sai Lee, Jiyun Park, Kyung-Soo Kim, Soo-Kyung Kim, Yong-Wook Cho, Young Shin Song
-
Endocrinol Metab. 2023;38(4):445-454. Published online July 18, 2023
-
DOI: https://doi.org/10.3803/EnM.2023.1702
-
-
 Abstract Abstract
 PDF PDF Supplementary Material Supplementary Material PubReader PubReader  ePub ePub
- Background
Papillary thyroid carcinoma (PTC) can be classified into two distinct molecular subtypes, BRAF-like (BL) and RASlike (RL). However, the molecular characteristics of each subtype according to clinicopathological factors have not yet been determined. We aimed to investigate the gene signatures and tumor microenvironment according to clinicopathological factors, and to identify the mechanism of progression in BL-PTCs and RL-PTCs.
Methods
We analyzed RNA sequencing data and corresponding clinicopathological information of 503 patients with PTC from The Cancer Genome Atlas database. We performed differentially expressed gene (DEG), Gene Ontology, and molecular pathway enrichment analyses according to clinicopathological factors in each molecular subtype. EcoTyper and CIBERSORTx were used to deconvolve the tumor cell types and their surrounding microenvironment.
Results
Even for the same clinicopathological factors, overlapping DEGs between the two molecular subtypes were uncommon, indicating that BL-PTCs and RL-PTCs have different progression mechanisms. Genes related to the extracellular matrix were commonly upregulated in BL-PTCs with aggressive clinicopathological factors, such as old age (≥55 years), presence of extrathyroidal extension, lymph node metastasis, advanced tumor-node-metastasis (TNM) stage, and high metastasis-age-completeness of resection- invasion-size (MACIS) scores (≥6). Furthermore, in the deconvolution analysis of tumor microenvironment, cancer-associated fibroblasts were significantly enriched. In contrast, in RL-PTCs, downregulation of immune response and immunoglobulin-related genes was significantly associated with aggressive characteristics, even after adjusting for thyroiditis status.
Conclusion
The molecular phenotypes of cancer progression differed between BL-PTC and RL-PTC. In particular, extracellular matrix and cancer-associated fibroblasts, which constitute the tumor microenvironment, would play an important role in the progression of BL-PTC that accounts for the majority of advanced PTCs.
|